Quality assurance creates systematic frameworks using documented procedures, trained personnel, and validated processes. These ensure consistent output that meets predetermined specifications.
What is Quality Assurance
Quality assurance is a systematic process that builds confidence in your products and services. It ensures they consistently meet specified requirements and exceed customer expectations.
The US Code of Federal Regulations offers a formal definition: quality assurance encompasses all planned and systematic actions necessary to provide confidence that a product or service will satisfy given requirements for quality. But here’s what sets it apart from quality control.
Quality control detects defects in finished products. The principles of quality assurance, however, take a preventive approach. They build quality into every production and service delivery stage. Think of it this way: QC catches problems after they happen. QA stops them before they start.
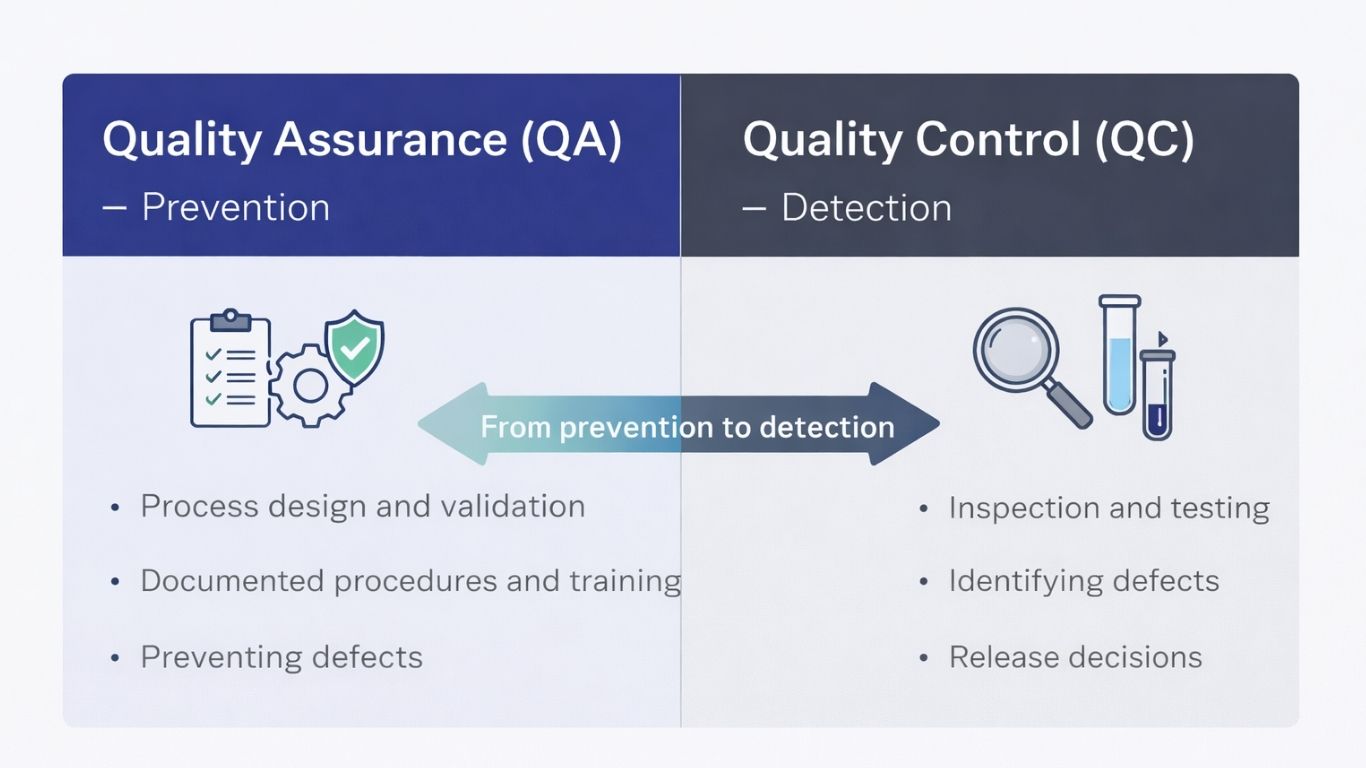
This distinction matters in timing and focus. Quality assurance operates proactively. It concentrates on preventing errors through proper planning, documentation, and process control. Quality control reacts. It identifies defects through post-production inspection and testing.
Why does this matter financially? Preventing defects costs far less than detecting and correcting them after manufacturing. Organizations implementing robust quality assurance systems see reduced waste, minimized rework, and enhanced customer satisfaction.
Here’s a fundamental truth: quality cannot be inspected into a product. It must be built in from conception through delivery.
The Critical Role in Regulated Industries
The pharmaceutical and biotechnology sectors treat quality assurance as non-negotiable. Their products directly impact human health and safety. Regulatory bodies like the FDA and European Medicines Agency mandate comprehensive quality assurance programs. These must comply with current Good Manufacturing Practice (GMP) requirements.
Regulated industries face strict release criteria. They cannot distribute products without demonstrating adherence to quality standards through detailed documentation, testing protocols, and batch records.
But quality assurance extends beyond mere compliance. Modern systems integrate risk management, continuous improvement methodologies, and data-driven decision making. They provide structured approaches for identifying potential problems, implementing corrective actions, and preventing recurrence through root cause analysis.
The Seven Principles of Quality Assurance
The seven principles of quality assurance form the bedrock of effective quality management systems across industries. ISO 9001:2015 establishes these quality management principles. They guide organizations in building robust frameworks.
These basic principles of quality assurance work universally. Whether you manufacture pharmaceuticals, produce medical devices, or deliver services, they apply.
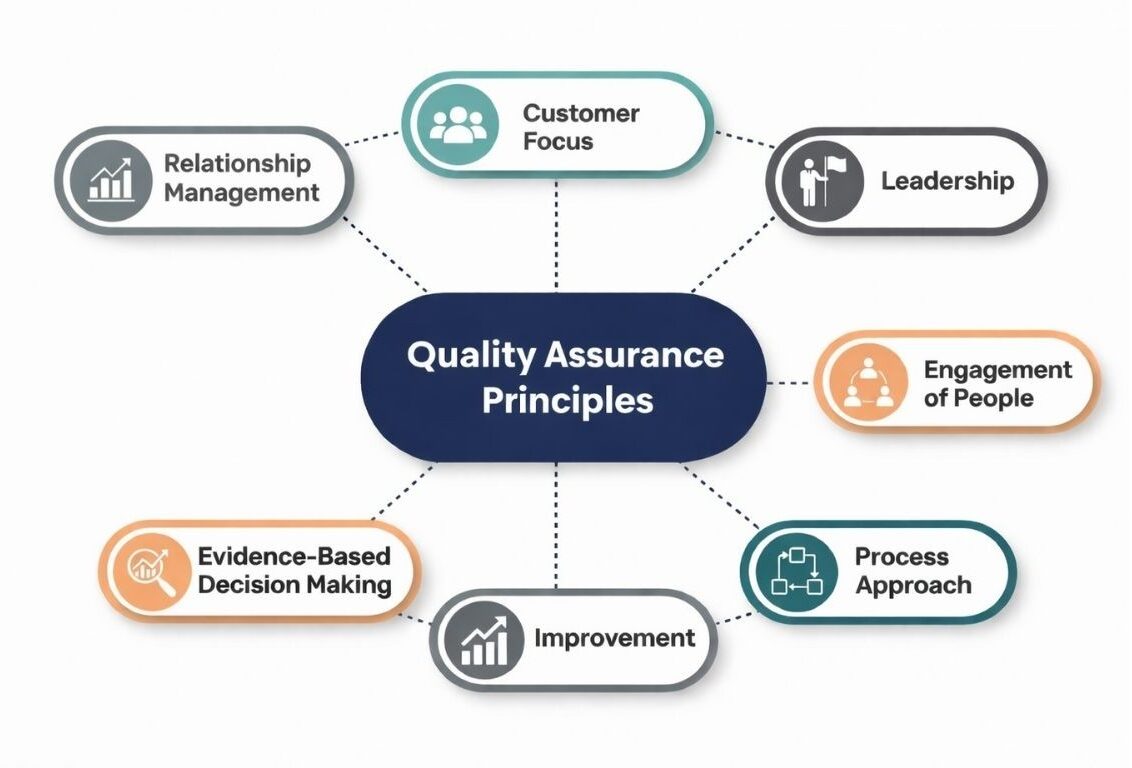
1. Customer Focus
This represents the first and foremost principle of quality assurance and performance improvement. Organizations exist to serve customers. Their satisfaction drives long-term success.
Customer focus requires understanding current and future customer needs. You must link those requirements to organizational objectives. Then evaluate processes to ensure they consistently deliver products meeting or exceeding expectations.
Companies achieving genuine customer focus see tangible results: increased repeat business, enhanced brand reputation, and sustainable competitive advantage.
2. Leadership
Strong leadership establishes organizational direction. It creates environments where people pursue quality objectives with purpose.
Effective leaders unify departments around quality goals. They demonstrate visible commitment to excellence. What does this look like practically? Providing adequate resources for quality assurance activities. Setting clear quality policies. Fostering accountability at every organizational level.
3. Engagement of People
Competent, empowered employees drive quality outcomes. This principle recognizes their central role.
Organizations must determine necessary competencies for personnel affecting quality. Then ensure appropriate education and training. Create cultures where employees understand how their work contributes to quality objectives.
Motivated, skilled workers reduce errors, improve processes, and enhance organizational capability. It’s that straightforward.
4. Process Approach
This principle treats organizational activities as interconnected processes rather than isolated functions. The systematic view enables better resource management and more efficient operations.
The process approach employs the Plan-Do-Check-Act (PDCA) cycle. Organizations plan objectives, implement changes, evaluate results, and adjust based on findings. This iterative methodology supports continuous quality improvement.
5. Improvement
Organizations must continuously enhance their processes, products, and services. Static systems fail.
Companies apply improvement through various methods. Reducing risks. Seizing opportunities. Correcting nonconformities. The improvement principle drives organizations beyond meeting current standards toward achieving excellence.
6. Evidence-Based Decision Making
Decisions must rest on objective data, not assumptions. This principle demands rigor.
Organizations collect and analyze information from multiple sources. They employ statistical methods where appropriate and base conclusions on factual evidence. This approach reduces uncertainty, enables identification of trends and patterns, and supports effective problem resolution.
7. Relationship Management
No organization operates in isolation. You depend on suppliers, partners, and stakeholders for sustained success.
Effective relationship management develops strategic partnerships. It maintains transparent communication and aligns goals with business partners for mutual benefit. Managing supplier relationships effectively ensures consistent input quality and reduces supply chain risks.
The 5 P’s of Quality Assurance
Pharmaceutical and biotechnology industries employ the 5 P’s framework as fundamental components of Good Manufacturing Practice. This framework provides practical guidance for implementing quality assurance in regulated manufacturing environments.
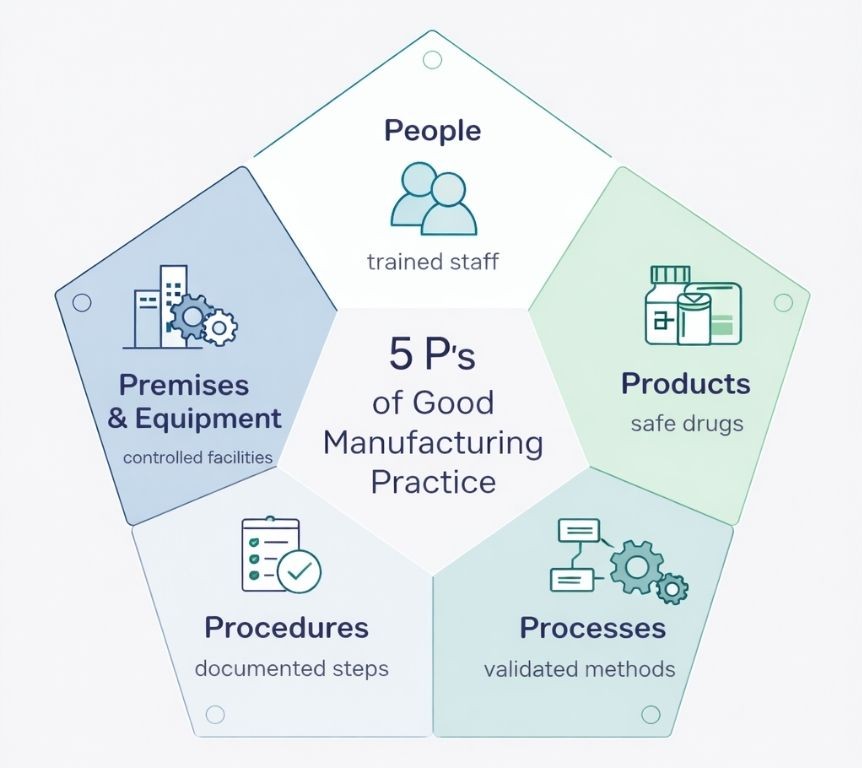
People
Qualified, trained personnel form the foundation of quality manufacturing. All employees must strictly adhere to manufacturing processes and regulations.
Comprehensive training programs cover specific roles and responsibilities. Organizations assess competency through performance evaluations and maintain detailed training records. Why? Because human error remains one of the greatest risks to product quality.
Products
Products require constant testing, comparison, and quality verification before distribution. Manufacturers establish clear specifications for all materials at every production phase.
A robust quality assurance system includes regular testing protocols, standard methods for packing and allocating samples, and comprehensive release criteria. Only conforming products reach customers.
Processes
Processes transform inputs into outputs. Each one must be clearly defined, validated, and controlled to achieve consistent results.
Organizations document processes comprehensively. They monitor critical parameters and employ statistical process control where appropriate. Process validation demonstrates that procedures consistently produce products meeting predetermined specifications.
Procedures
Standard Operating Procedures (SOPs) provide documented instructions for performing activities consistently. They detail step-by-step instructions for routine operations.
All employees must familiarize themselves with relevant procedures and follow them diligently. Deviations from SOPs require immediate reporting, investigation, and resolution.
Premises and Equipment
Facility design and equipment maintenance directly influence product quality. Manufacturing facilities require proper size, appropriate layout, and maintenance in good condition.
Environmental controls matter significantly. Temperature, humidity, and air quality must meet specifications, particularly in cleanroom environments. Regular equipment calibration, preventive maintenance, and qualification activities ensure reliable, consistent performance.
Operational Principles
Right First Time Philosophy
This philosophy emphasizes zero-defect manufacturing. Design and control processes to eliminate errors at the source.
The approach proves cost-effective. Why? The expense of quality problems increases exponentially as products move through production stages. Prevention beats correction every time.
Prevention vs. Detection
This core principle distinguishes quality assurance from quality control. Organizations invest in upstream activities: process design, validation, and control. These prevent defects rather than relying solely on downstream inspection to catch problems.
Preventive strategies include risk assessments, failure mode analysis, and robust process controls. They maintain operations within acceptable parameters.
Document Control
All procedures receive proper approval, regular review, and appropriate archiving. Master documents require version control, clear approval workflows, and accessibility to authorized personnel.
Documentation provides traceability. It supports regulatory compliance and enables effective training and knowledge transfer.
Compliance with Regulatory Standards
For industries like pharmaceuticals, medical devices, and food manufacturing, compliance is non-negotiable. Organizations must align quality assurance programs with applicable regulations.
This includes FDA 21 CFR Part 11 for electronic records, ISO 13485 for medical devices, and current Good Manufacturing Practice guidelines. Regulatory compliance requires understanding requirements, implementing controls, and maintaining evidence of conformance.
Auditing and Self-Inspection
Audits provide systematic mechanisms for verifying compliance and identifying improvement opportunities. Internal audits assess whether activities conform to planned arrangements. They verify whether the quality management system has been effectively implemented and maintained.
External audits by customers or certification bodies provide independent verification. They confirm system adequacy.
Validation and Verification
Processes and equipment must perform as intended. Process validation establishes documented evidence that procedures consistently produce products meeting predetermined specifications.
>> READ MORE: Verification and Validation Testing
Equipment qualification includes three phases:
- Installation Qualification (IQ): Confirms equipment is installed correctly
- Operational Qualification (OQ): Verifies equipment operates within specified limits
- Performance Qualification (PQ): Demonstrates consistent performance under actual conditions
Corrective and Preventive Action (CAPA)
CAPA systems investigate root causes of deviations and implement measures preventing recurrence. CAPA represents a systematic approach for collecting information, analyzing product and quality problems, and taking effective actions.
Organizations establish CAPA procedures defining problem identification, investigation methods, action planning, implementation, and effectiveness verification. The system transforms problems into opportunities for enhancement.
How to Achieve Consistent Quality Assurance
Establishing a Quality Management System
A quality management system (QMS) forms the cornerstone of achieving consistent quality assurance. To effectively apply the principles of quality assurance, organizations select appropriate frameworks.

ISO 9001 works for general quality management. ISO 13485 applies to medical devices. Industry-specific standards exist for other sectors.
The quality management system defines organizational structure, responsibilities, procedures, processes, and resources. Documentation hierarchy typically includes:
- Quality manual: Describes the overall quality management system
- Procedures: Detail how to perform activities
- Work instructions: Provide step-by-step guidance
- Records: Demonstrate compliance
Building a Quality Culture
Quality culture requires commitment from leadership, engagement of employees, and accountability throughout the organization. Leaders demonstrate visible commitment through resource allocation, policy setting, and personal participation in quality initiatives.
Organizations foster quality culture by recognizing and rewarding contributions. They empower people to identify and resolve issues. Most importantly, they promote understanding of how individual work affects overall quality objectives.
Implementing Standard Operating Procedures
SOPs ensure activities are performed consistently and correctly. Effective SOPs clearly describe procedures in sufficient detail for trained personnel to perform tasks correctly.
They use simple language. They avoid unnecessary technical jargon. They include appropriate references to related documents.
Organizations train personnel on SOPs, verify understanding through competency assessments, and maintain SOP currency through regular reviews.
Training and Competency Programs
Personnel must possess necessary skills and knowledge for their roles. Organizations determine competency requirements for each position affecting quality.
They provide appropriate education and training. They evaluate training effectiveness. They maintain records of competency. Ongoing education keeps personnel current with changing technologies, revised procedures, and evolving regulatory requirements.
Continuous Improvement Methodologies
Several structured approaches enhance quality assurance effectiveness:
Plan-Do-Check-Act (PDCA) enables organizations to test changes on small scales, evaluate results, and implement successful improvements.
Six Sigma employs statistical methods to reduce process variation and eliminate defects.
Lean manufacturing focuses on waste elimination and value stream optimization.
Kaizen promotes incremental improvements through employee involvement and suggestion systems.
Metrics and Key Performance Indicators
Measurement matters. Organizations track defect rates, customer complaints, audit findings, training completion rates, and CAPA effectiveness.
Regular analysis of quality metrics reveals trends. It highlights areas requiring attention and demonstrates the business value of quality investments.
Technology Integration
Technology enhances quality assurance through laboratory information management systems, quality management software, and automation. Electronic quality management systems centralize documentation and streamline workflows.
They provide real-time visibility into quality metrics. Automated systems reduce human error, improve consistency, and generate comprehensive audit trails supporting regulatory compliance.
Root Cause Analysis
Methodologies like the 5 Whys, fishbone diagrams, and failure mode effects analysis help organizations understand why problems occur. They don’t just address symptoms.
Effective root cause analysis requires gathering relevant data, examining all potential contributing factors, and identifying underlying systemic issues. Organizations then develop action plans addressing root causes rather than implementing superficial fixes.
Change Management
Modifications to materials, methods, or equipment must occur in controlled ways. Change control processes evaluate proposed changes for potential impacts on product quality.
They document justification for changes. They assess risks. They verify effectiveness of implemented modifications. Uncontrolled changes represent significant risks to product quality and regulatory compliance.
WHY CHOOSE US?
Implementing Quality Principles in Software Development
The principles of quality assurance extend beyond manufacturing into every industry demanding excellence, including software development. At HBLAB, we embody these principles through our CMMI Level 3 certification, demonstrating commitment to process excellence and continuous improvement.
Our quality assurance framework mirrors the seven ISO principles: customer focus drives our development methodology, leadership establishes clear quality objectives, and our 30% senior-level workforce ensures competent execution at every project phase.
Our Quality Assurance Approach:
We implement rigorous quality controls throughout the software development lifecycle. Our 700+ IT professionals follow documented Standard Operating Procedures, participate in ongoing competency training, and utilize CAPA systems to transform challenges into improvements. Prevention takes priority over detection, catching potential issues during design and development rather than after deployment.
Enterprise Quality Standards:
HBLAB maintains enterprise-grade security safeguards and IP protection protocols. We employ evidence-based decision making through metrics tracking, automated testing, and comprehensive audit trails. Our quality culture ensures consistent, reliable software delivery across all engagement models: offshore, onsite, dedicated teams, or Build-Operate-Transfer.
Business Value Through Quality:
Organizations partnering with HBLAB gain more than 30% cost efficiency compared to local rates. They access a quality management system that delivers consistent results without compromising standards. Our flexible engagement models maintain rigorous quality regardless of structure.
CONTACT US FOR A FREE CONSULTATION
Final Thoughts
The principles of quality assurance apply across industries. From manufacturing to healthcare. From technology to food production. Whether producing life-saving pharmaceuticals or delivering professional services, organizations benefit from systematic approaches.
These approaches prevent errors, ensure consistency, and drive continuous improvement. Implementing the principles of quality assurance represents an ongoing journey toward excellence. It requires commitment, discipline, and relentless focus on meeting and exceeding customer expectations while maintaining the highest standards of safety and efficacy.

